In the growing field of immunotherapy, Natural Killer (NK) cells have become a central focus for researchers and clinicians alike. These cells form a critical part of the body’s innate immune response, offering rapid defense against tumors and virally infected cells. Unlike other immune cells that require prior activation, NK cells act immediately—making them powerful candidates for therapeutic use. But their true potential in clinical applications depends on how efficiently they can be isolated, purified, and prepared for downstream use.
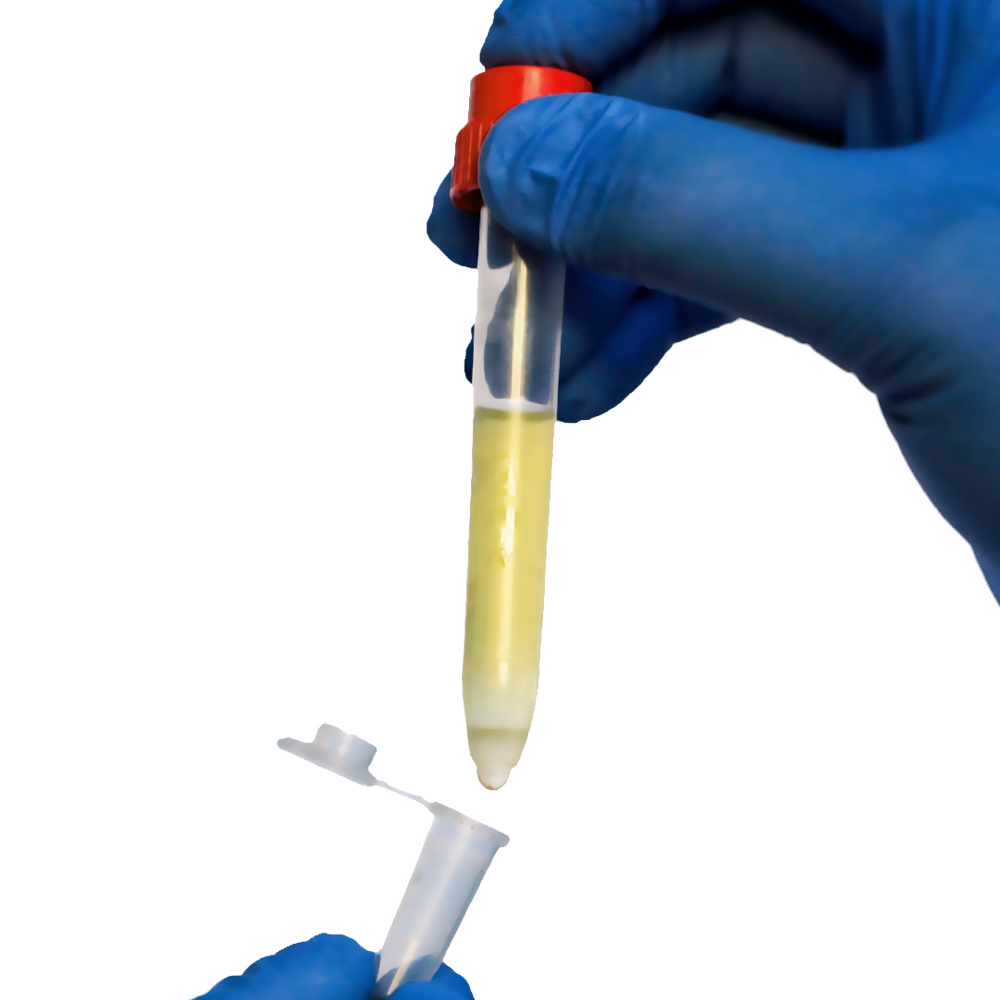
One of the essential steps in this preparation process is filtration. During the handling of blood, tissue, or peripheral blood mononuclear cell (PBMC) samples, unwanted debris, cell clumps, and non-target materials can reduce the quality of the final cell suspension. This is where Lab Cell Strainers come into play. By enabling the physical separation of target cells from impurities, strainers enhance the reliability and reproducibility of cell separation workflows.
Among available tools, SnapCap Lab Cell Strainers stand out due to their smart design and user-friendly operation. Integrated into standard lab tubes and built for sterile, closed handling, these strainers combine efficiency with ease of use—important for both research laboratories and cell therapy manufacturing settings.
This article takes a close look at how cell strainers, particularly the SnapCap format, contribute to the efficient enrichment of NK cells. From their role in supporting various particle separation techniques to their ability to preserve cell viability and improve workflow consistency, these tools are proving indispensable in the evolving space of immunotherapy research. Whether you’re optimizing NK cell sorting for flow cytometry or preparing cells for clinical-grade expansion, understanding the role of filtration tools like SnapCap strainers can bring both clarity and efficiency to your process.
Understanding NK Cells and Their Role in Immunotherapy
Natural Killer (NK) cells are a specialized subset of lymphocytes that play a frontline role in the immune system. Unlike T cells and B cells, which require antigen recognition to initiate a response, NK cells can act immediately upon encountering abnormal cells. This rapid action makes them especially valuable in detecting and destroying virally infected cells and certain types of cancer cells without prior sensitization.
One of the key functions of NK cells is their ability to recognize changes in the expression of major histocompatibility complex (MHC) class I molecules. Tumor cells and virally infected cells often downregulate MHC class I to evade detection by T cells—but this very change is what activates NK cells. Once triggered, NK cells release cytotoxic granules containing perforin and granzymes, inducing apoptosis in the target cells. They also secrete cytokines like IFN-γ that help coordinate the broader immune response.
In recent years, NK cells have gained significant interest in immunotherapy research. Their natural ability to target a wide range of abnormal cells makes them attractive candidates for both autologous and allogeneic cell therapies. Unlike T-cell-based therapies, NK cell therapies pose a lower risk of graft-versus-host disease, allowing for more flexibility in clinical applications.
However, to harness NK cells effectively, researchers must start with highly pure, functional cell populations. This requires precise and efficient cell separation workflows that remove debris and other cell types without damaging the NK cells themselves. Contaminants or clumps can reduce therapeutic efficacy and introduce unwanted variability in research data.
As a result, the use of filtration tools like Lab Cell Strainers becomes crucial early in the process. By providing clean, uniform suspensions, strainers help preserve NK cell viability and functionality, setting the foundation for more reliable enrichment and expansion steps that follow.
Why Cell Separation Is Critical for NK Cell Work
Isolating NK cells from a mixed population of blood cells is the first—and arguably most critical—step in any workflow involving Natural Killer (NK) cells. Whether the goal is to study their behavior in a research setting or prepare them for clinical immunotherapy, the success of downstream applications depends heavily on how well the cells are separated at the start.
Peripheral blood mononuclear cells (PBMCs) are often the starting point for NK cell isolation. This group includes a mix of lymphocytes (T cells, B cells, and NK cells), monocytes, and other cell types. Within this mixture, NK cells typically make up only 5–15% of the total PBMC population. Isolating them requires a process that is both precise and gentle to maintain cell function and viability.
Effective cell separation ensures that only the target NK cells are collected while excluding unwanted debris, dead cells, and aggregates that can affect purity. Impurities in the sample can interfere with downstream processes like cell expansion, flow cytometry, or therapeutic administration. For instance, T cells or monocytes remaining in the sample could influence cytokine profiles or skew assay results.
Another critical factor is the mechanical integrity of the cells. Harsh handling or clogged filtration tools can shear or damage the cells, compromising their cytotoxic function. That’s why pre-filtration using a Lab Cell Strainer is a valuable step—it removes clumps and large particles that could hinder later enrichment techniques like magnetic-activated cell sorting (MACS) or flow-based sorting.
In short, clean, debris-free, and unclumped suspensions are not just convenient—they’re essential. High-quality separation improves reproducibility, enhances the success of cell expansion protocols, and supports better outcomes in both experimental and clinical settings involving NK cells.
Common Particle Separation Techniques
To isolate NK cells effectively, labs rely on a series of particle separation techniques that reduce sample complexity, remove unwanted components, and concentrate the target population. Each technique contributes a specific function, and often, multiple methods are used in combination to achieve high purity and viability.
- Density Gradient Centrifugation:
This is a standard first step in many NK cell isolation protocols. Blood or bone marrow samples are layered over a medium like Ficoll and centrifuged. The process separates cells based on density, allowing peripheral blood mononuclear cells (PBMCs) to be collected at the interface layer. While this step enriches for lymphocytes, including NK cells, further refinement is still needed.
- Magnetic-Activated Cell Sorting (MACS):
MACS uses magnetic beads coated with antibodies specific to cell surface markers found on NK cells (e.g., CD56). After incubating the sample with these beads, cells are passed through a magnetic field. Labeled cells are retained or eluted, depending on the strategy used (positive or negative selection). This method is gentle, scalable, and widely adopted in clinical research.
- Fluorescence-Activated Cell Sorting (FACS):
FACS sorts cells one-by-one using laser-based detection of fluorescence-labeled antibodies. It provides extremely high purity and allows for sorting based on multiple markers simultaneously. While more complex and costly, it is ideal when a very specific NK cell subpopulation is needed.
- Cascade Straining and Pre-Filtration:
Before applying any of these advanced separation methods, physical filtration using Lab Cell Strainers is essential. Strainers with mesh sizes like 40 µm, 70 µm, or 100 µm help remove clumps and large debris. This pre-filtration step prevents clogging, ensures smooth flow through columns or cytometers, and preserves sample integrity.
Together, these techniques work in sequence to deliver clean, NK cell-enriched suspensions ready for downstream applications.
How Lab Cell Strainers Support Efficient NK Cell Enrichment
While advanced tools like MACS and FACS often take center stage in NK cell isolation, Lab Cell Strainers play an equally important role behind the scenes. These simple, yet essential devices help streamline the preparation of high-quality cell suspensions, making the entire enrichment workflow more effective and consistent.
The primary function of a Lab Cell Strainer is to remove clumps, debris, and aggregates from a sample before it undergoes further separation. In NK cell isolation, this filtration step is critical. Aggregated cells can interfere with magnetic separation columns, reduce the resolution of flow cytometers, or introduce variability in cell expansion protocols. By producing a uniform, single-cell suspension, strainers reduce these risks significantly.
Strainers such as the SnapCap Lab Cell Strainers go a step further. Designed with built-in mesh caps that fit directly into standard test tubes, they allow for one-handed operation, sterility, and airtight sealing. These features are especially useful when processing multiple samples in parallel, minimizing contamination and handling errors.
Mesh size also matters. For NK cell isolation, commonly used options like 40 µm, 70 µm, or 100 µm mesh strainers are ideal. A 70 µm mesh is particularly effective for removing large debris while retaining most lymphocytes, including NK cells. The ability to choose the right mesh size ensures better control over sample quality from the beginning.
Additionally, using cell strainers ahead of density gradients or magnetic sorting steps reduces the load on those systems. This translates into smoother operation, reduced column clogging, and better throughput—factors that are especially important in high-volume or clinical workflows.
In short, Lab Cell Strainers act as the quality gatekeepers in NK cell enrichment. They provide the physical separation foundation that enables high-precision, high-purity downstream isolation, which is essential for both research success and therapeutic reliability.
Integrating SnapCap with NK Cell Protocols
In workflows involving Natural Killer (NK) cell isolation, every step matters—from tissue dissociation to final enrichment. SnapCap Lab Cell Strainers integrate smoothly into these protocols, offering a simple yet effective solution to improve cell recovery and maintain consistency across batches.
The isolation of NK cells typically begins with the preparation of peripheral blood mononuclear cells (PBMCs) or other lymphoid tissue. This process often involves mechanical dissociation and density gradient centrifugation. Before applying any magnetic labeling or separation technique, researchers need to ensure the sample is free from clumps, large debris, and non-cellular matter. SnapCap strainers serve as a crucial pre-filtration step at this stage, helping to protect cell integrity and minimize blockages in downstream separation systems.
With a range of mesh sizes, SnapCap strainers can be selected based on specific needs. For example, a 70 µm cell strainer is often used post-dissociation to eliminate residual tissue fragments, while a 40 µm cell strainer may be preferred before flow cytometry or sorting procedures, ensuring a single-cell suspension. The integrated cap makes it easy to use these strainers directly on standard 15 ml or 50 ml tubes without needing additional adaptors or holders.
What sets SnapCap apart is its compatibility with both manual and automated systems. Whether processing samples by hand in small batches or scaling up for higher throughput, SnapCap strainers support seamless integration without disrupting existing workflows. Their leak-proof sealing is particularly beneficial when working with biohazardous materials, as it enhances safety and reduces handling errors.
By incorporating SnapCap strainers into NK cell isolation protocols, researchers can simplify filtration steps, reduce preparation time, and improve the purity of the resulting cell population. This streamlined integration supports more reliable results and enables labs to maintain high standards in both research and clinical environments.
Conclusion
Natural Killer (NK) cells are at the forefront of immunotherapy research due to their inherent ability to identify and destroy cancerous or virally infected cells. The quality of NK cell-based therapies, however, relies heavily on the precision of the upstream cell preparation process. One of the most overlooked yet critical components in this process is the cell strainer.
Lab Cell Strainers play a vital role in ensuring that the cell suspensions used for NK enrichment are clean, unclumped, and ready for accurate separation. Among the various strainer options available, SnapCap Lab Cell Strainers stand out for their practical, lab-ready design and performance. By combining a high-quality nylon mesh with a snap-lid integrated into a standard test tube format, SnapCap strainers make filtration easier, safer, and more consistent.
From supporting sterile conditions to offering mesh flexibility for different stages of NK cell isolation, these strainers are more than just passive tools—they are active contributors to workflow success. Their ability to handle varying sample types, from blood to tissue-derived suspensions, makes them highly adaptable for diverse research and clinical environments.
SnapCap Lab Cell Strainers also simplify the transition from manual to semi-automated or fully automated protocols, ensuring that as labs scale their NK cell research or therapies, their filtration tools can keep up. Whether used in academic labs, biotech companies, or clinical manufacturing settings, these strainers provide the reliability and ease of use needed for high-quality immunotherapy outputs.
Ultimately, enriching NK cells efficiently requires attention to detail at every step. With the inclusion of SnapCap Lab Cell Strainers in your protocol, you’re choosing a product that aligns with the needs of modern cell separation practices—helping researchers achieve better consistency, higher yields, and more effective immunotherapeutic outcomes.
 English
English French
French
 German
German
 Spanish
Spanish
 Belgium
Belgium
 Italian
Italian Brazil
Brazil Chinese Mandarin
Chinese Mandarin