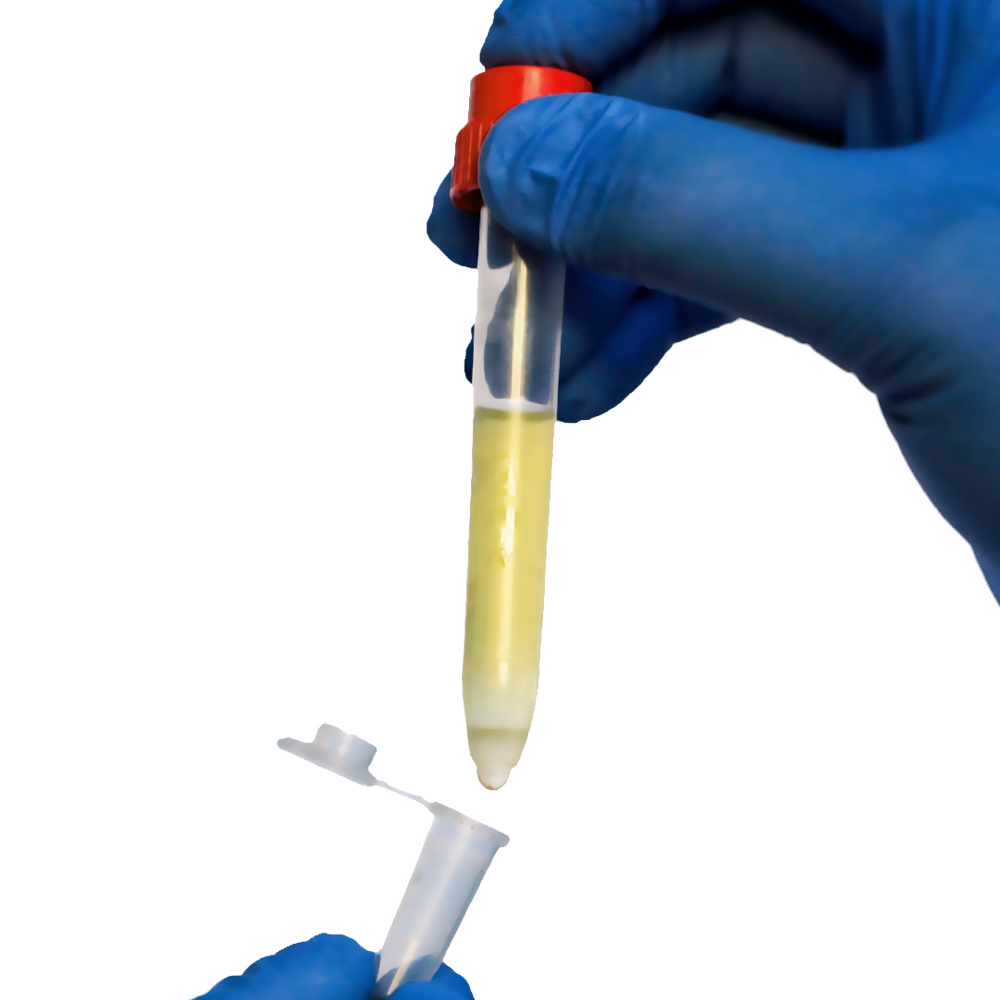
In cell-based research and applied laboratory workflows, the smallest steps often have the largest impact. Long before cells are enriched, labeled, sorted, or analyzed, they pass through a critical preparation phase. During this early stage, samples are filtered, clarified, and cleaned to remove debris, aggregates, or unwanted particles. For fragile cell types, such as stem cells, regulatory T cells, primary immune cells, or rare populations, this phase is where success is often decided.
Small-volume workflows may appear simple on paper, but in practice they are the most vulnerable to error, stress, and loss. Repeated centrifugation, manual mesh handling, pouring between containers, and excessive pipetting introduce mechanical stress, temperature shifts, evaporation, and variability. Each additional manipulation step increases the likelihood of damaging cells or losing material that cannot be replaced.
Minimizing sample manipulation is therefore not a convenience, it is a necessity. Protecting fragile cells requires tools that remove unnecessary steps, limit physical stress, and allow precise, controlled handling. The Pipette-Strainer was designed with exactly this goal in mind. By enabling two-way, pipette-based filtration for small volumes, it offers laboratories a gentler alternative to traditional preparation methods that rely heavily on centrifugation and transfers.
This article explores why minimizing manipulation matters, where traditional workflows add risk, and how the Pipette-Strainer helps preserve cell health, yield, and reproducibility across research and business laboratory environments.
Why Minimizing Sample Manipulation Matters
Cells are highly responsive to their environment. Mechanical forces, temperature changes, osmotic shifts, and prolonged exposure to non-optimal conditions all influence viability and function. While many downstream processes are optimized and carefully controlled, early preparation steps are often treated as routine, and this is where problems begin.
Each manipulation step introduces risk:
Mechanical stress
Mechanical forces introduced by centrifugation, fast pipetting, or uneven mesh contact can damage cell membranes and internal structures, reducing viability and altering normal cellular behavior.
Physiological stress
Extended exposure to non-ideal buffers, fluctuating temperatures, or open containers can trigger stress responses in cells, leading to apoptosis, activation, or unintended functional changes.
Sample loss
Transfers between tubes, pouring steps, and repeated aspiration increase the chance of losing cells, which is especially harmful when sample volume or cell numbers are limited.
Fragile cells are particularly sensitive. Stem cells may begin differentiating when exposed to stress, reducing their regenerative potential. Regulatory T cells may lose suppressive function or become activated. Primary immune cells can suffer reduced viability, impacting downstream assays and reproducibility.
In both research and applied settings, these changes translate into lower yields, inconsistent results, and wasted time. Minimizing manipulation protects not only the cells themselves but also the integrity of the entire workflow.
The Hidden Risks of Traditional Small-Volume Filtration
Many laboratories rely on conventional preparation methods that were originally designed for larger volumes. When adapted to small samples, these methods introduce inefficiencies and risks that are often underestimated.
Repeated Centrifugation
Centrifugation is widely used to clarify suspensions or remove debris, but it is not a neutral process. Even short spins subject cells to high forces, and repeated cycles amplify the stress. Centrifugation also adds waiting time, requires access to shared equipment, and introduces variability depending on rotor type, speed, and balancing accuracy. For dense suspensions or fine mesh filtration, centrifugation becomes a bottleneck rather than a solution.
Manual Mesh Handling and Pouring
Traditional mesh strainers often require samples to be poured into a strainer placed on top of a tube. For small volumes, this step is particularly risky. Meshes can shift, liquid can splash, and even a single lost drop can represent a significant portion of the sample. Re-aligning strainers mid-process further increases handling and contamination risk.
Multiple Transfers Between Containers
Every transfer, from tube to strainer, from strainer to new tube, from centrifuge tube to pipette, adds another opportunity for loss. Transfers also change concentration subtly, especially when evaporation or incomplete recovery occurs.
Operator Variability
Manual steps depend heavily on technique. Differences in pipetting speed, angle, pressure, or timing can lead to inconsistent results between operators or batches. In workflows that process many samples per day, this variability becomes a serious limitation.
The Need for a Gentler, More Controlled Alternative
What fragile cell workflows need is not another complex system, but fewer steps. Reducing manipulation means reducing stress, loss, and variability. The ideal preparation tool should:
Allow direct filtration without pouring
Direct pipette-based filtration removes the need to pour samples between containers, reducing spills, loss, and uncontrolled movement during preparation.
Reduce or eliminate centrifugation
By replacing centrifugation with pipette-driven filtration, samples avoid high forces, saving time while protecting fragile cells from mechanical stress.
Support precise, controlled movement
Controlled aspiration and dispensing through the strainer allows gentle handling, ensuring consistent flow and minimizing shear forces on sensitive cells.
Work efficiently with small volumes
Designed specifically for low-volume samples, the system maintains accuracy and recovery where traditional strainers struggle to perform reliably.
Integrate seamlessly into existing workflows
Compatibility with standard pipettes allows immediate adoption without new equipment, retraining, or changes to established laboratory protocols.
The Pipette-Strainer was designed to meet these needs.
Introducing the Pipette-Strainer
The Pipette-Strainer is a two-way filtration device optimized for small volumes. Unlike traditional strainers that rely on gravity and pouring, it integrates directly with pipettes, allowing filtration to occur during aspiration or dispensing.
At the core of its design is a perforated elastomer top assembled into the strainer housing. This elastomer creates high friction between the pipette and the strainer, enabling stable, step-by-step handling. The result is controlled filtration with minimal physical stress.
The Pipette-Strainer is available in two formats:
- Pipette-Strainer-T, designed for standard 1–5 mL pipette tips
- Pipette-Strainer-S, designed for 1–10 mL serological pipettes
Both versions share the same functional principle: reduce filtration time and handling steps in situations where centrifugation is traditionally used, especially with small mesh sizes or dense suspensions.
How Pipette-Strainer Reduces Sample Manipulation
Two-Way Filtration Through the Pipette
One of the most significant advantages of the Pipette-Strainer is its two-way functionality. Samples can be filtered as they are aspirated or dispensed through the pipette. This eliminates the need to pour samples into a separate device or reposition containers during processing. By keeping the sample within a single, controlled flow path, the Pipette-Strainer reduces the number of open steps and minimizes exposure to the environment.
High-Friction Elastomer for Gentle Control
The specially designed elastomer top provides friction that stabilizes the pipette during filtration. This allows users to move liquid slowly and evenly across the mesh, avoiding sudden pressure changes that can damage cells or force debris through the filter. Controlled movement is particularly important for fragile cells, where shear stress can compromise viability or surface markers.
Reduced Dependence on Centrifugation
For many small-volume workflows, centrifugation is used simply because no better alternative exists. The Pipette-Strainer reduces the need for centrifugation by enabling efficient filtration even with dense suspensions or fine meshes. By replacing centrifugation with pipette-based filtration where appropriate, labs save time and protect cells from unnecessary force.
Fewer Transfers, Higher Recovery
Because filtration happens directly through the pipette into the receiving vessel, transfers between containers are minimized. This directly improves recovery, especially when working with limited or irreplaceable samples.
Pipette-Strainer-T vs. Pipette-Strainer-S: Choosing the Right Format
While both versions share the same core benefits, choosing the right format ensures optimal workflow integration.
Pipette-Strainer-T (1–5 mL Pipette Tips)
The T-version is ideal for precision work with small volumes. It integrates seamlessly with standard pipette tips commonly used in research labs. This makes it particularly suitable for:
- Small-volume cell suspensions
- Rare cell populations
- Pilot experiments and assay development
- Fine control during step-by-step preparation
Pipette-Strainer-S (1–10 mL Serological Pipettes)
The S-version is designed for slightly larger volumes and workflows involving cell and tissue culture. Its compatibility with serological pipettes makes it well-suited for:
- Creating single-cell suspensions from tissue digests
- Filtering culture media or supplements
- Handling viscous or dense suspensions
- Situations where gentle flow is required without repeated centrifugation
Both versions support the same goal: reducing manipulation while maintaining control.
Benefits for Fragile Cell Types
Stem Cells
Stem cells are highly sensitive to mechanical and environmental stress. Excessive centrifugation or rough handling can trigger differentiation or reduce viability. By enabling gentle, controlled filtration with fewer steps, the Pipette-Strainer helps maintain stem cell phenotype and functional potential. This controlled preparation is especially valuable during early-stage processing, where stress can have lasting downstream effects. Consistent handling also improves reproducibility across experiments and donor samples.
Regulatory T Cells (Tregs)
Tregs require careful handling to preserve their suppressive function. Mechanical stress or prolonged processing can lead to activation or functional loss. The Pipette-Strainer reduces handling time and avoids harsh forces, supporting more reliable preparation before downstream enrichment or analysis. Shorter exposure times help maintain physiological conditions during preparation. This results in more stable populations for functional assays and immune studies.
Primary Immune Cells
Primary immune cells are often obtained in limited numbers and must remain viable for functional assays. Minimizing transfers and centrifugation helps preserve these cells and improves consistency across samples. Gentle filtration also reduces variability caused by operator technique. This is particularly important when comparing results across multiple donors or experimental runs.
Rare Cell Populations
When working with rare populations, every cell counts. The Pipette-Strainer’s design minimizes loss during preparation, helping ensure that more target cells reach downstream steps intact. Reduced handling lowers the chance of accidental depletion or contamination. This makes the device especially useful for workflows where recovery directly impacts experimental success.
Improving Reproducibility Across Operators and Batches
One often overlooked benefit of reduced manipulation is improved reproducibility. Workflows with fewer manual steps are inherently more consistent. The Pipette-Strainer standardizes filtration by integrating it into a familiar tool, the pipette, reducing technique-dependent variation.
This is particularly valuable in laboratories that process many samples daily or operate across multiple sites. Consistency in early preparation steps supports more reliable downstream results and easier troubleshooting.
Integrating Pipette-Strainer into Cell Separation and Enrichment Workflows
Although the Pipette-Strainer is a preparation tool, it plays an important supporting role in broader workflows involving cell separation and enrichment. Clean, debris-free suspensions improve the performance of downstream processes, whether they involve labeling, binding, or density-based steps.
By clarifying samples early and gently, the Pipette-Strainer helps protect cell surface markers and reduces interference from debris or aggregates. This leads to smoother downstream processing and more consistent outcomes.
Saving Time Without Sacrificing Quality
In addition to protecting cells, minimizing manipulation saves time. Eliminating centrifugation cycles, reducing transfers, and streamlining filtration allows labs to process more samples in less time. For business-focused laboratories, this translates into higher throughput and lower operational costs.
Importantly, these time savings do not come at the expense of quality. On the contrary, gentler handling often improves results by preserving cell health and reducing variability.
A Practical Upgrade for Everyday Lab Work
The Pipette-Strainer does not require new infrastructure, complex training, or workflow redesign. It fits naturally into existing laboratory routines, replacing steps that add risk without adding value.
For labs seeking incremental but meaningful improvements in efficiency, yield, and reproducibility, it represents a practical upgrade rather than a disruptive change.
Conclusion
Protecting fragile cells starts long before enrichment or analysis begins. It starts with preparation. By minimizing sample manipulation at this early stage, laboratories can preserve cell health, improve recovery, and achieve more reliable and reproducible results across experiments and batches. Each unnecessary transfer, centrifugation step, or manual adjustment introduces stress that can compromise sensitive cell populations and reduce overall yield.
The Pipette-Strainer enables a meaningful shift away from these force-based, transfer-heavy workflows. By allowing filtration to occur directly through the pipette, it removes several common handling steps that traditionally slow workflows and increase risk. Its two-way design supports smooth, step-by-step filtration, while the high-friction elastomer top provides stability and control during handling. Compatibility with both standard pipette tips and serological pipettes ensures that it fits naturally into existing laboratory routines without added complexity.
For laboratories working with small volumes, delicate cell types, or limited samples, the Pipette-Strainer is more than a filtration device. It is a practical workflow improvement that helps safeguard cell integrity, reduce variability, and support consistent downstream performance—protecting what matters most: the cells themselves.
 English
English French
French
 German
German
 Spanish
Spanish
 Belgium
Belgium
 Italian
Italian Brazil
Brazil Chinese Mandarin
Chinese Mandarin