 B cell isolation and stimulation with pluriBeads
B cell isolation and stimulation with pluriBeads
Isolation
| Sample Material |
50ml EDTA whole blood |
| Insulation method |
Non-magnetic with 300 µl CD19 S-pluriBeads® anti-human |
| Yield |
~3.5 * 10^6 B lymphocytes |
| Vitality |
>99% (Trypan blue stain) |
| Purity determination |
Anti-CD20 FITC antibody staining followed by FACS analysis. |
|
|
Analysis
Frequency determination of antigen-specific memory B cells.
After target activation, the abundance of antibody-secreting plasma cells (ASC) was determined by ELISpot.
Isolated B cells were stimulated with a polyclonal activation mixture for 5 days. They were then grown on a plate coated with the specific antigen. After 24 h incubation, the assay was performed.
The spots were counted and evaluated with a special software (AID ELISpot6.0 iSpot). In the adjacent diagram, the blue numbers indicate the number of antigen-specific spots per well.
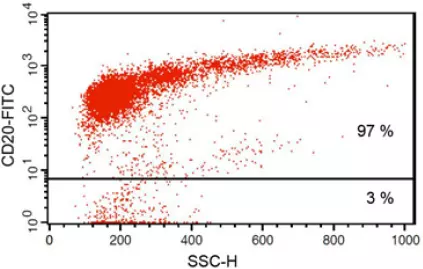
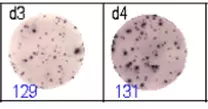
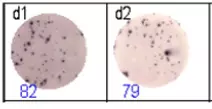
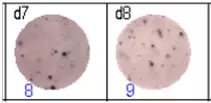
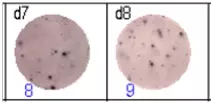
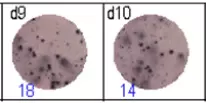
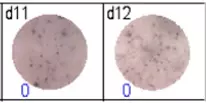

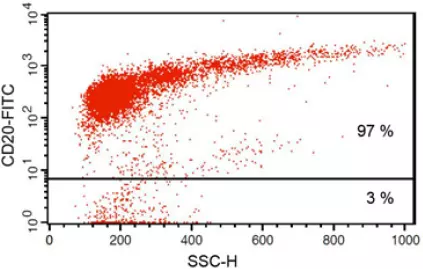

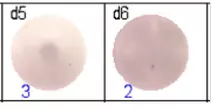

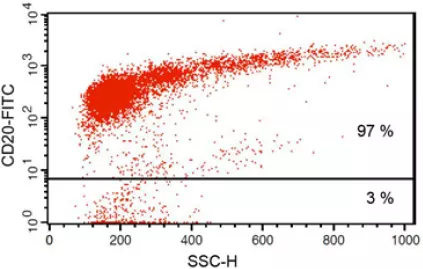

Fig 1: Purity of isolated & stimulated B lymphocytes
Fig 2: ELISpot analysis of anti-Ig specific cells in duplicates. d1d2: 250 cells/well each, d3d4: 500 cells/well each, d5d6: non-activated cells (500 cells/well each)
Fig 3: d7d8: Anti-influenza nucleocapsid-specific memory B cells, d9d10: nti tetanus toxoid-specific memory B cells, d11d12: negative control.
Evaluation / Summary
S-pluriBead® CD19 enabled the isolation of B lymphocytes from a human whole blood sample with a purity of ~ 97%.
The isolated targets showed >99% viability after trypan blue staining. They were successfully grown in cell culture as well as differentiated into antibody-secreting plasma cells (ASCs). ASCs were found to be suitable for analysis of antigen-specific memory B cell response by ELISpot assay. The results were comparable to those obtained with a reference method (Dynabeads® CD19 Pan B).
For the reference method, see publication: Bussmann BM, Reiche S, Bieniek B, Krznaric I, Ackermann F, Jassoy C: Loss of HIV-specific memory B-cells as a potential mechanism for the dysfunction of he humoral immune response against HIV. Virology 2010; 397(1):7-13.)