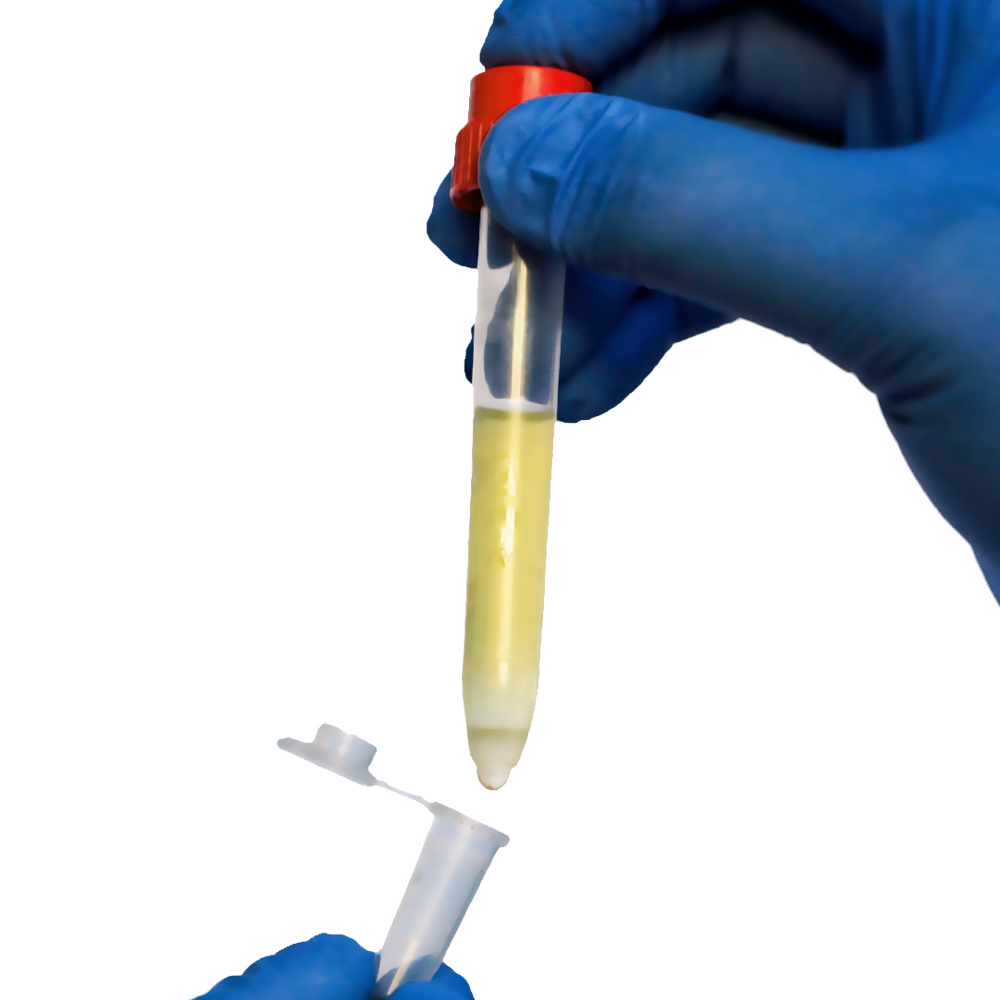
Discover how pluriBead technology transforms cell separation, focusing on monocyte isolation and dendritic cell maturation, pivotal for advancing immunological research and therapeutic applications.
In the realm of life sciences, the precise isolation and enrichment of specific cell types from complex biological mixtures are pivotal for advancing both research and clinical applications. This process is crucial in understanding cellular mechanisms, developing therapies, and enhancing diagnostic techniques.
Monocyte isolation and the subsequent maturation into dendritic cells are particularly significant in immunology and medical research. Dendritic cells are key players in the immune system, responsible for presenting antigens to T-cells and initiating immune responses. Thus, efficiently isolating and maturing these cells is critical for studying immune responses, vaccine development, and immunotherapy.
PluriBead technology represents an innovative approach to a non-magnetic antibody supported cell separation. This innovative method offers researchers a reliable and efficient means to isolate monocytes and facilitate their transformation into mature dendritic cells. By harnessing the power of pluriBead technology, scientists can achieve high purity and yield, paving the way for advancements in immunological research and therapeutic applications. In this article, we’ll explore how pluriBead technology works, its application in monocyte isolation for dendritic cell maturation, and why it stands out as the best antibody cell separation technology for researchers and clinicians.
Understanding pluriBead Technology
pluriBead technology utilizes non-magnetic, monodispersed microparticles (beads) that are coated with monoclonal antibodies (mAb) specific to target cell surface structures. These beads serve as a platform for the selective binding and isolation of target cells from complex mixtures, such as whole blood or cell suspensions.
The technology operates through a straightforward process: during incubation with the sample material, the target cells adhere to the pluriBeads® due to the specific antibody-antigen interactions. This binding allows for subsequent separation using a pluriStrainer, which employs size exclusion to isolate the bead-bound target cells from the suspension.
Unlike traditional methods that may require pretreatment steps like density centrifugation or erythrolysis to enhance target cell concentration, pluriBead® technology eliminates the need for such preparations. This simplifies and accelerates the cell separation process, making it more efficient and accessible for researchers and clinicians alike.
Key Features and Benefits
- Selective Binding: Monoclonal antibodies on pluriBeads ensure precise targeting and binding of desired cells, enhancing purity and yield.
- Efficiency: Rapid and effective isolation of target cells directly from complex samples without extensive pre-processing.
- Versatility: Suitable for various applications in immunology, cancer research, regenerative medicine, and beyond, accommodating different sample types and cell sizes.
- User-Friendly: Simplified workflow with minimal hands-on time and straightforward protocols, reducing experimental variability and ensuring reproducibility.
Comparison with Traditional Cell Separation Methods
PluriBead® technology offers several advantages over traditional methods such as fluorescence-activated cell sorting (FACS) or magnetic-activated cell sorting (MACS):
No Magnetic Interference: Unlike MACS, pluriBeads are non-magnetic, preventing interference with magnetic-sensitive assays and allowing for broader compatibility in downstream applications.
Gentle on Cells: The gentle binding and detachment process of pluriBeads® preserves cell viability and functionality, critical for downstream cell culture and analysis.
Cost-Effective: Eliminates the need for expensive equipment like flow cytometers or magnetic separators, reducing overall experimental costs while maintaining high performance.
In summary, pluriBead technology represents a significant advancement in cell separation methods, offering researchers a reliable, efficient, and cost-effective solution for isolating and enriching specific cell populations essential for various biomedical and clinical research applications.
Why Monocyte Isolation is Important
Monocytes play a crucial role in the immune system, serving as key components in innate immunity and as precursors to dendritic cells, which are pivotal in adaptive immunity.
Applications of Monocyte-Derived Dendritic Cells in Research and Therapy
Monocyte-derived dendritic cells (moDCs) are specialized antigen-presenting cells crucial for initiating and regulating adaptive immune responses. They capture antigens, process them into smaller fragments, and present these antigens on their cell surface in conjunction with major histocompatibility complex (MHC) molecules. This antigen presentation is essential for activating T cells and initiating specific immune responses against pathogens or cancer cells.
In research, moDCs are valuable tools for studying immune responses, vaccine development, and understanding diseases like cancer, autoimmune disorders, and infectious diseases. Researchers can manipulate moDCs in vitro to study their interaction with pathogens or to develop therapeutic strategies aimed at enhancing immune responses against tumors or infections.
In therapy, moDCs hold promise for immunotherapy approaches, where they can be loaded with specific antigens or tumor-associated antigens to stimulate anti-tumor immune responses. This approach, known as dendritic cell-based immunotherapy, is being explored for its potential to treat various cancers and other immunological disorders effectively.
Overall, the isolation and study of monocytes, particularly their differentiation into dendritic cells, provide critical insights into immune function and offer promising avenues for both research and therapeutic applications aimed at combating diseases and enhancing immune responses.
Step-by-Step Guide: Monocyte Isolation with pluriBead®
Preparation of Sample Material and Buffers
Before beginning the isolation process with pluriBead®, it is essential to prepare the necessary sample material and buffers:
1. Sample Material:
Collect whole blood, buffy coat, or any other relevant cell suspension containing the desired target cells, such as monocytes.
2. Buffers:
- Wash Buffer (10x Stock Solution): This buffer is used for washing steps to remove unwanted components and debris from the sample.
- Buffer A (Stabilization Buffer): A chelating agent that helps preserve the integrity of the blood sample.
- Buffer B (Incubation Buffer): Used to adjust the density of the sample, particularly useful for isolating peripheral blood mononuclear cells (PBMCs).
- Buffer C (Detachment Activation Buffer): Activates the detachment process of target cells from pluriBeads®.
- Buffer D (Detachment Concentrate): Facilitates the detachment of target cells directly on the pluriStrainer®.
Incubation Process with pluriBead® and Sample Material
Mixing and Incubation:
- Combine the prepared sample material with pluriBeads® coated with monoclonal antibodies (mAb) specific to the target cell surface markers.
- Gently mix the sample and pluriBeads® and incubate at room temperature. During this incubation period, the target cells in suspension will bind to the pluriBeads® through specific antibody-antigen interactions
Duration of Incubation:
- Follow the manufacturer’s guidelines or experimental protocols for the recommended incubation time, typically ranging from 15 to 30 minutes, ensuring optimal binding of target cells to pluriBeads®.
Washing and Detachment of Target Cells from pluriBead
Sieving with pluriStrainer:
- After the incubation period, transfer the mixture onto a pluriStrainer, which facilitates the separation of pluriBeads with bound target cells from the rest of the sample. The pluriStrainer is designed to retain bead-bound target cells while allowing other cells and debris to pass through.
Detachment Process:
- Apply Buffer C (Detachment Activation Buffer) to activate the detachment of target cells from pluriBeads directly on the pluriStrainer.
- Use Buffer D (Detachment Concentrate) to complete the detachment process, ensuring efficient recovery of detached cells into a fresh collection tube.
Detailed Protocol for Monocyte Isolation, Differentiation, and Stimulation
1. Isolation of Monocytes:
- Post-detachment, isolated monocytes can be further processed for differentiation into dendritic cells or other desired cell types.
- Determine the cell count and purity using appropriate methods, such as flow cytometry or cell counting techniques.
2. Differentiation and Stimulation:
- Resuspend the isolated monocytes in a suitable culture medium, such as RPMI 1640 supplemented with FCS and antibiotics.
- Follow a specific differentiation and stimulation protocol tailored to the experimental needs, which may involve incubation with cytokines (e.g., GM-CSF and IL-4) to induce dendritic cell maturation.
- Monitor cell morphology, viability, and marker expression during the culture period to assess differentiation efficiency.
-
This step-by-step guide outlines the process of monocyte isolation using pluriBead, emphasizing the critical steps from sample preparation to the differentiation and stimulation of isolated cells for various research applications in immunology, cancer biology, and therapeutic development.
Conclusion
In conclusion, pluriBead technology represents a significant advancement in the field of cell separation, particularly in the isolation of monocytes for dendritic cell maturation. By leveraging monoclonal antibody-coated microparticles, pluriBead simplifies and accelerates the process of isolating specific cell populations from complex samples without the need for extensive pre-processing steps. This technology not only enhances the efficiency and reliability of cell isolation but also preserves cell viability and functionality critical for downstream applications.
The maturation of dendritic cells plays a pivotal role in immunology and therapeutic research. Monocyte-derived dendritic cells, generated through pluriBead-facilitated isolation, hold promise for applications in cancer immunotherapy, vaccine development, and autoimmune disease treatments. By understanding and optimizing the conditions for dendritic cell maturation, researchers can harness the immune system’s potential to combat diseases effectively.
Moving forward, continued research and innovation in pluriBead antibody cell separation technology and dendritic cell biology will pave the way for new discoveries and therapeutic strategies. As we strive towards enhancing immune responses and personalized medicine, pluriBead remains a valuable tool for researchers and clinicians alike, driving advancements in biomedicine and improving patient outcomes worldwide.
Discover how pluriBead technology can enhance your research and therapeutic applications today. Contact Pluriselect-USA for product inquiries, and demonstrations, and to learn more about integrating pluriBead® into your laboratory workflows.
 English
English French
French
 German
German
 Spanish
Spanish
 Belgium
Belgium
 Italian
Italian Brazil
Brazil Chinese Mandarin
Chinese Mandarin