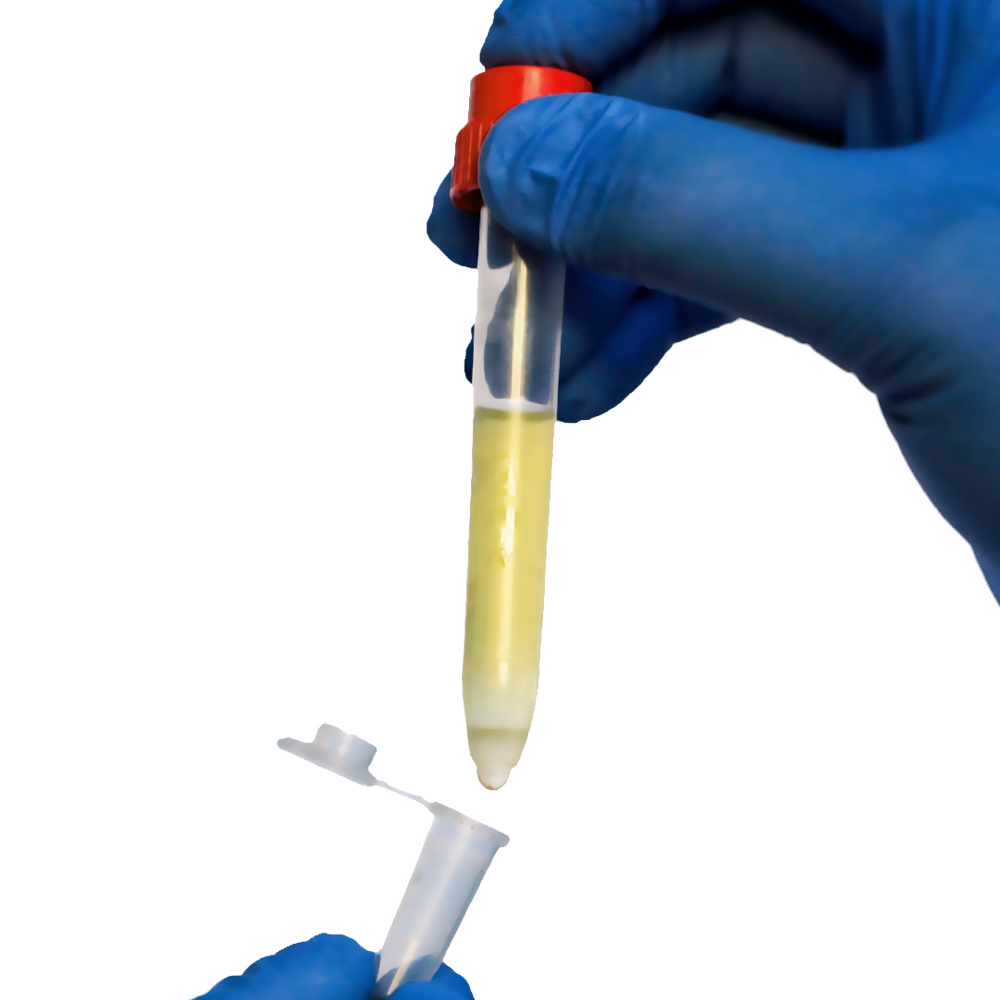
A buffy coat is an accumulation of platelets and white blood cells (WBCs). We’ll discuss how TwinSpin helps in the preparation of a buffy coat.
Blood is made up of millions of cells suspended in plasma. These cells include platelets, white blood cells, and red blood cells, which carry oxygen and fight infection (which help with clotting). Due to their abundance—thousands of red blood cells can be seen in a single drop of blood—examination of red blood cells is straightforward.
On the other hand, white blood cells are present in very small amounts and are more challenging to examine. The quickest way to look at a lot of white blood cells is to examine a buffy coat smear. All of the white blood cells and platelets in a blood sample make up the buffy coat.
We will also find out how TwinSpin density gradient centrifugation tubes are used in preparation of Buffy Coat From Whole Blood.
A Buffy Coat: What Is It?
White blood cells (WBCs) and platelets make up less than 1% of the cells in a sample of peripheral whole blood. These WBCs and platelets combine to form their own layer that is suspended between the red blood cells (RBCs) and supernatant plasma when researchers spin the blood sample in a centrifuge. Due to its color, this thin layer is referred to as a buffy coat (yellowish to brownish).
The buffy coat has a 10–20X higher concentration of leukocytes than the rest of the sample, which are white blood cells that aid in the body’s ability to fight off infection. Researchers working in toxicology or patients with platelet or WBC dysfunction may find this to be helpful.
A Brief On Twinspin Tubes
TwinSpin centrifuge tubes with PBMC already inside Peripheral blood mononuclear cells (PBMC) can be isolated from bone marrow and whole blood using Spin Medium. The TwinSpin is made up of an inner tube and a standard 15. The inner tube’s open bottom is buried in the density gradient medium (DGM). The blood sampling tube can be directly pipetted into the TwinSpin with anticoagulated blood or bone marrow.
The sample is placed on top of the DGM inside the inner tube. Leukocytes, lymphocytes, and PBMCs are separated from unwanted erythrocytes and granulocytes during density gradient centrifugation, depending on the density gradient used (Leuko Spin, PBMC Spin, PBMC 24+, or PLT Spin Medium).
Target cells above the DGM are therefore enriched in the interphase. Through the DGM, the erythrocytes will exit the inner tube and fall to the bottom of the outer tube. Once the inner tube has completely separated, just take it out.
As a valve, the elastic cap is used. By removing the cap, the collection tube can be transformed into a pipette. One drop at a time can be removed to collect the contents.
Pre-filled Density Gradient Medium
- Leuko Spin is used to separate leukocytes from bone marrow or whole blood.
- PBMC Spin: Used to separate PBMC from bone marrow or whole blood.
- PBMC 24+ Spin – For the separation of PBMC from bone marrow or whole blood older than 12 hours.
- PLT Spin is used to separate platelets from bone marrow or whole blood.
Instructions for Using the TwinSpin Tube
- Ensure that the (diluted blood) sample, the TwinSpin, and the centrifuge are all at room temperature.
1.1 Check that the inner tube is partially filled and immersed in the DGM. If not, maintain the vertical position while rotating the TwinSpin device.
1.2 Take out and throw away the transport stopper
- Include Sample Material
2.1 Pipette the sample material on top of the DGM in the inner tube by tilting the TwinSpin 45° and allowing the sample to run down the inside of the dropper.
2.2 Push the elastic cap firmly into the opening to close the TwinSpin. PluriSpin PLT Depletion can be used to reduce platelet contamination.
- Spin
3.1 Centrifuge at 800 x g for 20 minutes at room temperature in a swing bucket rotor, brake off. If the blood is more than 4 hours old, centrifuge it for 30 minutes at 1000g.
- Collect
4.1 Remove the inner cell separation tube by unscrewing it.
4.2 Push the elastic cup down to collect cells in the opaque layer in a new tube.
5 Wash (if necessary)
5.1 Add wash buffer to the reaction tube.
5.2 Cells are spun down for 10 minutes with 300 x g at 4 °C (no or small break).
5.3 Remove the supernatant and set the reaction tube on the table for 20 seconds.
Excess wash buffer will flow down the tube wall and accumulate at the bottom. Aspirate the majority of the liquid above the pellet. The liquid will appear foggy because it contains mostly platelets; aspiration will improve the washing result.
5.5 Carefully pipette the pellet with 1 ml of wash buffer.
5.6 Re-do steps 5.1–5.4.
5.7 Refill the pellet to the desired volume.
Preparation of buffy coat from scratch
Buffy coats can be prepared in two different ways. To separate the whole blood into red cells, plasma, and buffy coat, the whole blood donation is centrifuged on the one hand. The second option is to filter the blood and keep the leukocytes from circulating.
- Preparing a Buffy Coat fraction in your lab using fresh whole blood
- Another option is to create the buffy coat on your own. So just adhere to this quick protocol:
- Combine one part washing buffer with one part whole blood.
- Using the brake off, centrifuge the diluted whole blood for 10 minutes at 200 x g.
- Remove the interphase leukocytes (buffy coat)
Further Applications
With PluriBead, Buffy Coat can be used to separate specific cell types:
- High cell counts in a short period of time
- Unprepared samples
- Perfect for separating uncommon cell types
- Following isolation, it is simple to recover the sample material, enabling the isolation of more target cells.
You can go to our website for more details. Use our products to experience the distinction for yourself. Start using our density gradient centrifugation products for cell separation right away!
Reference:
NCBI
Science Direct
Parasites And Vectors
 English
English French
French
 German
German
 Spanish
Spanish
 Belgium
Belgium
 Italian
Italian Brazil
Brazil Chinese Mandarin
Chinese Mandarin